Page 216 - Haematologica - Vol. 105 n. 6 - June 2020
P. 216
I. Scheller et al.
ABC
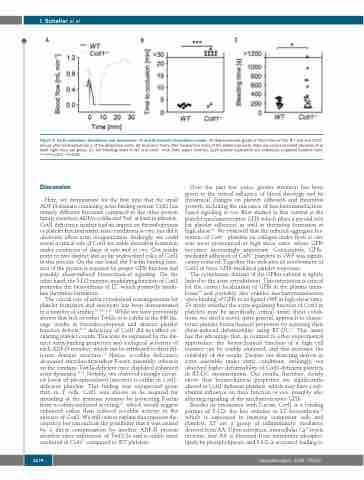
Figure 5. Cotl1 modulates thrombosis and hemostasis. (A and B) Intravital thrombosis model. (A) Representative graph of blood flow of one WT and one Cotl1-/- mouse after mechanical injury of the abdominal aorta. (B) Occlusion times after mechanical injury of the abdominal aorta. Data are mean±standard deviation of at least eight mice per group. (C) Tail bleeding times in WT and Cotl1-/- mice (filter paper method). Each symbol represents one individual. Unpaired Student t-test: ***P<0.001; *P<0.05.
Discussion
Here, we demonstrate for the first time that the small ADF-H-domain-containing actin-binding protein Cotl1 has entirely different functions compared to the other protein family members ADF/n-cofilin and Twf, at least in platelets. Cotl1 deficiency neither had an impact on thrombopoiesis or platelet function under static conditions in vitro, nor did it obviously affect actin reorganization. Strikingly, we could reveal a critical role of Cotl1 for stable thrombus formation under conditions of shear in vitro and in vivo. Our results point to two distinct and so far undescribed roles of Cotl1 in this process. On the one hand, the F-actin binding func- tion of the protein is required for proper GPIb function and possibly shear-induced biomechanical signaling. On the other hand, the 5-LO enzyme-modulating function of Cotl1 promotes the biosynthesis of LT, which positively modu- late thrombus formation.
The crucial role of actin cytoskeletal rearrangements for platelet formation and reactivity has been demonstrated in a number of studies.5,7,8,10,11,39 While we have previously shown that lack of either Twf2a or n-cofilin in the MK lin- eage results in thrombocytopenia and distinct platelet function defects,10,11 deficiency of Cotl1 did not affect cir- culating platelet counts. This may be explained by the dis- tinct actin-binding properties and biological activities of each ADF-H member, which can be attributed to their dif- ferent domain structure.12 Hence, n-cofilin deficiency decreased stimulus-dependent F-actin assembly, whereas on the contrary, Twf2a-deficient mice displayed enhanced actin dynamics.10,11 Notably, we observed strongly elevat- ed levels of phosphorylated (inactive) n-cofilin in Cotl1- deficient platelets. This finding was unexpected given that, in T cells, Cotl1 was shown to be required for spreading at the immune synapse by protecting F-actin from n-cofilin-mediated severing,14 which would suggest enhanced rather than reduced n-cofilin activity in the absence of Cotl1. We still cannot explain this apparent dis- crepancy but can exclude the possibility that it was caused by a direct compensation by another ADF-H protein member since expression of Twf1/2a and n-cofilin were unaltered in Cotl1-/- compared to WT platelets.
Over the past few years, greater attention has been given to the critical influence of blood rheology and its dynamical changes on platelet adhesion and thrombus growth, including the relevance of mechanotransduction- based signaling in vivo. Best studied in this context is the platelet mechanoreceptor GPIb which plays a pivotal role for platelet adhesion, as well as thrombus formation at high shear.24 We observed that the reduced aggregate for- mation of Cotl1-/- platelets on collagen under flow in vitro was most pronounced at high shear rates, where GPIb becomes increasingly important. Consistently, GPIb- mediated adhesion of Cotl1-/- platelets to vWF was signifi- cantly reduced. Together, this indicates an involvement of Cotl1 in basic GPIb-mediated platelet responses.
The cytoplasmic domain of the GPIbα subunit is tightly linked to the actin cytoskeleton. This interaction is critical for the correct localization of GPIb in the plasma mem- brane40 and probably also enables mechanotransduction upon binding of GPIb to its ligand vWF at high shear rates. To study whether the actin-regulating function of Cotl1 in platelets may be specifically critical under shear condi- tions, we used a novel, quite general, approach to charac- terize platelet biomechanical properties by assessing their shear-induced deformability using RT-DC.27 This assay has the advantage that, in contrast to other experimental approaches, the biomechanical function of a high cell number can be readily analyzed, and this increases the reliability of the results. Despite not detecting defects in actin assembly under static conditions, strikingly, we observed higher deformability of Cotl1-deficient platelets in RT-DC measurements. Our results, therefore, clearly show that biomechanical properties are significantly altered in Cotl1-deficient platelets, which may have a sub- stantial influence on their function in vivo, possibly also affecting signaling of the mechanoreceptor GPIb.
Besides its interaction with F-actin, Cotl1 is a binding partner of 5-LO, the key enzyme in LT biosynthesis,15 which is expressed in immune competent cells and platelets. LT are a group of inflammatory mediators derived from AA. Upon activation, intracellular Ca2+ levels increase, free AA is liberated from membrane phospho- lipids by phospholipases, and 5-LO is activated, leading to
1674
haematologica | 2020; 105(6)