Page 18 - Haematologica - Vol. 105 n. 6 - June 2020
P. 18
Editorials
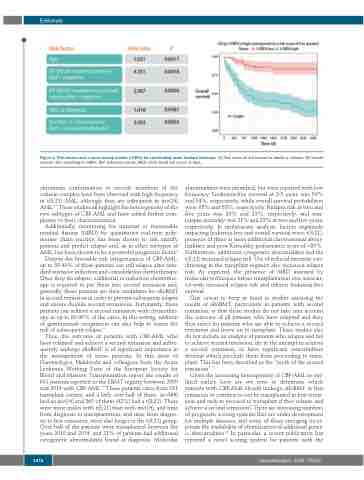
Figure 1. Risk factors and a novel scoring system (I-CBFit) for core-binding acute myeloid leukemia. (A) Risk ratios of risk factors for death or relapse. (B) Overall survival (OS) according to I-CBFit. Ref: reference values; WBC: white blood cell count; d: days.
chromatin conformation or encode members of the cohesin complex have been observed with high frequency in t(8;21) AML, although they are infrequent in inv(16) AML.6,7 These studies all highlight the heterogeneity of the two subtypes of CBF-AML and have added further com- plexity to their characterization.
Additionally, monitoring for minimal or measurable residual disease (MRD) by quantitative real-time poly- merase chain reaction has been shown to risk stratify patients and predict relapse and, as in other subtypes of AML, has been shown to be a powerful prognostic factor.8
Despite the favorable risk categorization of CBF-AML, up to 30-40% of these patients can still relapse after stan- dard intensive induction and consolidation chemotherapy. Once they do relapse, additional re-induction chemother- apy is required to put them into second remission and, generally, these patients are then candidates for alloBMT in second remission in order to prevent subsequent relapse and ensure durable second remissions. Fortunately, these patients can achieve a second remission with chemother- apy in up to 80-90% of the cases. In this setting, addition of gemtuzumab ozogamicin can also help to lessen the risk of subsequent relapse.9
Thus, the outcome of patients with CBF-AML who have relapsed and achieve a second remission and subse- quently undergo alloBMT is of significant importance in the management of these patients. In this issue of Haematologica, Halaburda and colleagues from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation report the results of 631 patients reported to the EBMT registry between 2000 and 2014 with CBF-AML.10 These patients came from 181 transplant centers, and a little over half of them (n=366) had an inv(16) and 265 of them (42%) had a t(8;21). There were more males with t(8;21) than with inv(16), and time from diagnosis to transplantation, and time from diagno- sis to first remission, were also longer in the t(8;21) group. Over half of the patients were transplanted between the years 2010 and 2014, and 21% of patients had additional cytogenetic abnormalities found at diagnosis. Molecular
abnormalities were identified, but were reported with low frequency. Leukemia-free survival at 2-5 years was 59% and 54%, respectively, while overall survival probabilities were 65% and 58%, respectively. Relapse risk at two and five years was 20% and 23%, respectively, and non- relapse mortality was 21% and 23% at two and five years, respectively. In multivariate analysis, factors negatively impacting leukemia-free and overall survival were: t(8;21), presence of three or more additional chromosomal abnor- malities, and poor Karnofsky performance score of <80%. Furthermore, additional cytogenetic abnormalities and the t(8;21) increased relapse risk. Use of reduced intensity con- ditioning in the transplant regimen also increased relapse risk. As expected, the presence of MRD assessed by molecular techniques before transplantation was associat- ed with increased relapse risk and inferior leukemia-free survival.
One caveat to keep in mind in studies assessing the results of alloBMT, particularly in patients with second remission, is that these studies do not take into account the outcome of all patients who have relapsed and they thus select for patients who are able to achieve a second remission and move on to transplant. These studies also do not include an analysis of patients who relapse and fail to achieve second remission, die in the attempt to achieve a second remission, or have significant comorbidities develop which preclude them from proceeding to trans- plant. This has been described as the “myth of the second remission”.11
Given the increasing heterogeneity of CBF-AML as out- lined earlier, how are we now to determine which patients with CBF-AML should undergo alloBMT in first remission or continue to not be transplanted in first remis- sion and only to proceed to transplant if they relapse and achieve a second remission? There are increasing numbers of prognostic scoring systems that are under development for multiple diseases, and some of those emerging incor- porate the availability of identification of additional genet- ic abnormalities.12 In particular, a recent publication has reported a novel scoring system for patients with the
1476
haematologica | 2020; 105(6)