Page 13 - Haematologica - Vol. 105 n. 6 - June 2020
P. 13
Editorials
identified activation of p53 pathway, induction of apopto- sis, and downregulation of MYC expression, thus func- tionally demonstrating restoration of wild-type p53 func- tion. Notably, transcriptome analysis with confirmation by RT-qPCR also identified a novel synergistic mechanism of FLT3 pathway downregulation. Importantly, the inhibi- tion of cell proliferation with combination therapy could be overcome in a dose-dependent fashion in the presence of FLT3 ligand, highlighting a novel therapeutic mecha- nism of APR-246 that could potentially be exploited in combination with FLT3 inhibitors in future clinical study.
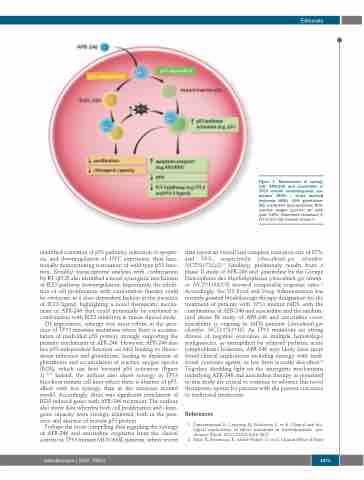
Of importance, synergy was most robust in the pres- ence of TP53 missense mutations where there is accumu- lation of misfolded p53 protein, strongly supporting the primary mechanism of APR-246. However, APR-246 also has p53-independent function via MQ binding to thiore- doxin reductase and glutathione, leading to depletion of glutathione and accumulation of reactive oxygen species (ROS), which can feed forward p53 activation (Figure 1).18,19 Indeed, the authors also show synergy in TP53 knockout mutant cell lines where there is absence of p53, albeit with less synergy than in the missense mutant model. Accordingly, there was significant enrichment of ROS-induced genes with APR-246 treatment. The authors also show data whereby both cell proliferation and clono- genic capacity were strongly inhibited, both in the pres- ence and absence of mutant p53 protein.
Perhaps the most compelling data regarding the synergy of APR-246 and azacitidine originates from the clinical activity in TP53 mutant MDS/AML patients, where recent
Figure 1. Mechanisms of synergy with APR-246 and azacitidine in TP53 mutant myelodysplastic syn- dromes (MDS) / acute myeloid leukemia (AML). GSH: glutathione; MQ: methylene quinuclidinone; ROS: reactive oxygen species; wt: wild- type; TrxR1: thioredoxin reductase 1; FLT-3: fms like tyrosine kinase 3.
data report an overall and complete remission rate of 87% and 53%, respectively (clinicaltrials.gov identifier: NCT03072043).9 Similarly, preliminary results from a phase II study of APR-246 and azacitidine by the Groupe Francophone des Myélodysplasies (clinicaltrials.gov identifi- er: NCT03588078) showed comparable response rates.10 Accordingly, the US Food and Drug Administration has recently granted breakthrough therapy designation for the treatment of patients with TP53 mutant MDS with the combination of APR-246 and azacitidine and the random- ized phase III study of APR-246 and azacitidine versus azacitidine is ongoing in MDS patients (clinicaltrials.gov identifier: NCT03745716). As TP53 mutations are strong drivers of negative outcomes in multiple hematologic malignancies, as exemplified by relapsed pediatric acute lymphoblastic leukemia, APR-246 may likely have more broad clinical implications including synergy with tradi- tional cytotoxic agents, as has been recently described.20 Together, shedding light on the synergistic mechanisms underlying APR-246 and azacitidine therapy as presented in this study are critical to continue to advance this novel therapeutic option for patients with the poorest outcomes to traditional treatments.
References
1. Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and bio- logical implications of driver mutations in myelodysplastic syn- dromes. Blood. 2013;122(22):3616-3627.
2. Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical Effect of Point
haematologica | 2020; 105(6)
1471