Page 297 - Haematologica May 2020
P. 297
Testosterone and post-transplant outcome of men
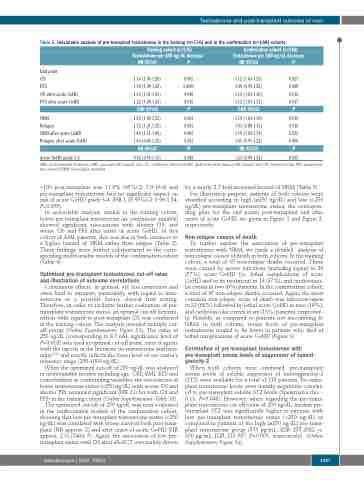
Table 2. Univariable analysis of pre-transplant testosterone in the training (n=176) and in the confirmation (n=168) cohorts.
Training cohort (n=176) Testosterone per 100 ng/dL decrease
Confirmation cohort (n=168) Testosterone per 100 ng/dL decrease
End point
OS
PFS
OS after acute GvHD PFS after acute GvHD
NRM
Relapse
NRM after acute GvHD Relapse after acute GvHD
Acute GvHD grade 3-4
HR 95%CI
1.16 (1.05-1.28) 1.18 (1.08-1.28) 1.19 (1.01-1.41) 1.22 (1.04-1.43) CHR 95%CI
1.28 (1.09-1.52) 1.12 (1.01-1.25) 1.45 (1.11-1.89) 1.06 (0.86-1.32) HR 95%CI
0.92 (0.74-1.15)
P
0.002 0.0005 0.040 0.015 P
0.003 0.033 0.005 0.552 P
0.448
HR 95%CI
1.12 (1.03-1.25) 1.09 (0.99-1.20) 1.15 (1.03-1.30) 1.12 (1.01-1.23) CHR 95%CI
1.19 (1.03-1.30) 1.01 (0.88-1.15) 1.19 (1.03-1.39) 1.05 (0.91-1.22) HR 95%CI
1.23 (0.99-1.54)
P
0.027 0.088 0.012 0.037 P
0.019 0.916 0.022 0.490 P
0.055
AML: acute myeloid leukemia; CHR: cause-specific hazard ratio; CI: confidence interval; GvHD: graft-versus-host disease; HR: hazard ratio; OS: overall survival; PFS: progression- free survival; NRM: non-relapse mortality.
+100 post-transplant was 11.9% (95%CI: 7.0-16.8) and pre-transplant testosterone had no significant impact on risk of acute GvHD grade 3-4 (HR 1.23 95%CI: 0.99-1.54, P=0.055).
In univariable analysis, similar to the training cohort, lower pre-transplant testosterone (as continuous variable) showed significant associations with shorter OS, and worse OS and PFS after onset of acute GvHD. In this cohort of AML patients, this was due in both instances to a higher hazard of NRM rather than relapse (Table 2). These findings were further substantiated in the corre- sponding multivariable models of the confirmation cohort (Table 4).
Optimized pre-transplant testosterone cut-off value and illustration of outcome correlations
Continuous effects, in general, are less instructive and often hard to interpret, particularly with regard to inter- ventions in a possible future clinical trial setting. Therefore, in order to facilitate further evaluation of pre- transplant testosterone status, an optimal cut-off determi- nation with regard to post-transplant OS was conducted in the training cohort. The analysis revealed multiple cut- off points (Online Supplementary Figure S3). The value of 250 ng/dL (corresponding to 8.7 nM, significance level of P=0.018) was used as optimal cut-off point, since it agrees with the reports in the literature on testosterone and mor- tality15,16 and exactly reflects the lower level of our center’s reference range (250-1000 ng/dL).
When the optimized cut-off of 250 ng/dL was analyzed in multivariable models including age, CRP, BMI, KPS and comorbidities as confounding variables, the associations of lower testosterone status (<250 ng/dL) with worse OS and shorter PFS remained significant (HR 2.0 for both OS and PFS) in the training cohort (Online Supplementary Table S8).
The optimized cut-off of 250 ng/dL was next evaluated in the multivariable models of the confirmation cohort, showing that low pre-transplant testosterone status (<250 ng/dL) was correlated with worse survival both post trans- plant (HR approx. 2) and after onset of acute GvHD (HR approx. 2.3) (Table 5). Again, the association of low pre- transplant status with OS after alloSCT was mainly driven
by a nearly 2.7-fold increased hazard of NRM (Table 5). For illustration purpose, patients of both cohorts were stratified according to high (≥250 ng/dL) and low (<250 ng/dL) pre-transplant testosterone status; the correspon- ding plots for the end points post-transplant and after onset of acute GvHD are given in Figure 1 and Figure 2,
respectively.
Non-relapse causes of death
To further explore the association of pre-transplant testosterone with NRM, we made a detailed analysis of non-relapse causes of death in both cohorts. In the training cohort, a total of 35 non-relapse deaths occurred. These were caused by severe infections (including sepsis) in 20 (57%), acute GvHD (i.e. lethal complications of acute GvHD and/or its treatment) in 13 (37%), and cardiovascu- lar events in two (6%) patients. In the confirmation cohort, a total of 47 non-relapse deaths occurred. Again, the most common non-relapse cause of death was infection/sepsis in 32 (68%) followed by lethal acute GvHD in nine (19%), and cardiovascular events in six (13%) patients, respective- ly. Notably, as compared to patients not succumbing to NRM, in both cohorts, serum levels of pre-transplant testosterone tended to be lower in patients who died of lethal complications of acute GvHD (Figure 3).
Correlation of pre-transplant testosterone with pre-transplant serum levels of suppressor of tumori- genicity-2
When both cohorts were combined, pre-transplant serum levels of soluble suppressor of tumorigenicity-2 (ST2) were available for a total of 218 patients. Pre-trans- plant testosterone levels were weakly negatively correlat- ed to pre-transplant soluble ST2 levels (Spearman's rho: - 0.13, P=0.048). However, when regarding the pre-trans- plant testosterone cut-off value of 250 ng/dL, median pre- transplant ST2 was significantly higher in patients with low pre-transplant testosterone status (<250 ng/dL) as compared to patients of the high (≥250 ng/dL) pre-trans- plant testosterone group (573 pg/mL, IQR 255-2082 vs. 350 pg/mL, IQR 212-587, P=0.005, respectively) (Online Supplementary Figure S4).
haematologica | 2020; 105(5)
1457