Page 24 - Haematologica May 2020
P. 24
M. Engelhardt et al.
The aim of this commentary is to define strategies in MM patients, and explore how frailty assessment may be employed in clinical practice and clinical trials.
Instruments to assess vulnerability due to increased treatment options
The epidemiologic and biologic considerations of elderly MM patients, with widely expanding treatment options, have motivated global efforts to validate simple instru- ments to assess vulnerability of patients, test them in their clinical significance to predict treatment outcome [overall survival (OS) and progression-free survival (PFS)], occur- rence of severe adverse events, and to tailor treatment with more or less intensified regimens.11,12,18
Under-treatment of fit elderly patients has been demonstrated to occur more frequently than over-treat- ment.12 Under-treatment may prevent improvement of organ function, while over-treatment of frail patients can induce unnecessary morbidity and mortality. Both instances reduce patients' health-related quality of life (HRQOL). In a study that assessed HRQOL across >16,000 cancer survivors, those with MM were among those with the lowest HRQOL scores, highlighting the
urgent need for this to be improved and for frequent reassessment of HRQOL in cancer patients.19 The art of managing elderly MM patients involves balancing com- peting disease-related and patient-specific factors and to make adequate treatment decisions.
Numerous induction (and relapse) MM-treatment options are available today. These include bortezomib- cyclophosphamide-dexamethasone (VCD), bortezomib- lenalidomide-dexamethasone (VRD or VTD), borte- zomib-melphalan-prednisone (VMP) or antibody-combi- nations, autologous stem cell transplantation (ASCT) and 2-drug combinations, such as lenalidomide-dexametha- sone (Rd), bortezomib-dexamethasone (Vd), and oth- ers.3,20-22 These largely expanded therapeutic strategies, including immunotherapies,23 have significantly evolved in recent years, but the beneficial effect is not seen across the age spectrum, with intermediate-fit or frail patients not obtaining the maximal benefit from such new treat- ment. Part of this failure to achieve benefit relates to the host biology of older patients. Therefore, there is an unmet need to give the right therapy to the patient most suited to benefit from it; the starting point for this approach is an appropriate classification of who is fit and who is frail.
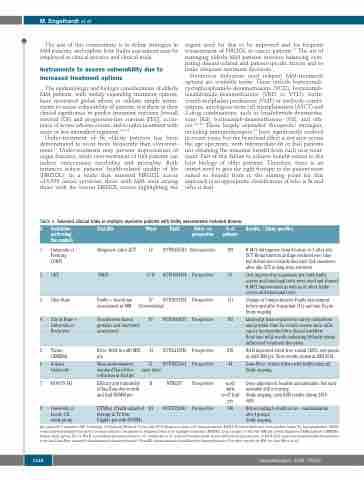
Table 1. Selected clinical trials in multiple myeloma patients with frailty assessments included therein.
# Institution Trial title performing
the analysis
1 University of Allogeneic (allo)-SCT Freiburg
(UKF)
2 UKF VBDD
3 Ohio State Frailty + functional assessment in MM
Phase
IV
I / II
IV Observational
Retro- vs. prospective
Retrospective
Prospective
Prospective
Prospective
Prospective
Prospective
Prospective
Prospective
Trial#
NCT00655343
NCT01394354
N. of patients
109
33
111
165
210
44
n=65
unfit, n=67 frail pts
740
Results / Study specifics
R-MCI did improve from 4 before to 3 after allo- SCT. Renal function and age declined over time, but did not neccessarily decrease QoL measures after allo-SCT in long-term survivors.
QoL improved in responsive pts: both frailty scores and functional tests were used and showed R-MCI improvement as well as of other frailty scores and functional tests.
Change in Comprehensive Frailty Assessment: before and after transplant (Tx) and non-Tx pts. Study ongoing.
Limited pt time required for survey completion and provider time for results review show mGA can be incorporated into clinical workflow. Real-time mGA results indicating fit/frailty status influenced treatment decisions.
4 City of Hope + Touchscreen-based IV University of geriatric and functional Rochester assessment
NCT02033928
NCT03068637
NCT02215980
NCT04223661
NTR6297
NCT03720041
5 Torino, Rd vs. Rd-R in unfit MM GIMEMA pts
6 Indiana Maia randomization: University standard Dara-Rd vs. reduction in frail pts
7 HOVON 143 Efficacy and tolerability of Ixa-Dara-dex in unfit
and frail NDMM pts
II
II, open label
II
III
Rd-R improved event-free survial (EFS) end points in unfit MM pts. First results shown at ASH 2018.
Dara-Rd vs. reduced dose with frailty index ≥2. Study ongoing.
8 University of FiTNEss (Frailty-adjusted Leeds, UK therapy in Tx Non-
study group Eligible pts with NDMM)
Dose-adjustment feasible and advisable, but early mortality still occurring.
Study ongoing, early ASH results shown 2019: #695.
IRd according to frailty score - randomization into 4 groups.
Study ongoing.
pts: patients, #: number, UKF: University of Freiburg Medical Center, allo-SCT: allogeneic stem cell transplantation, R-MCI: Revised Myeloma Comorbidity Index; Tx: transplantation; VBDD: vorinostat-bortezomib-doxorubicin-dexamethason treatment in relapsed/refractory multiple myeloma (RRMM), QoL: Quality of life; ND MM pts: newly diagnosed MM patients; GIMEMA: Italian study group. Rd vs. RD-R: Lenalidomide-dexamethason vs. continuation of reduced lenalidomide doses without dexamethason in Rd-R, IRd: ixazomib-lenalidomide-dexametha- sone; Ixa-Dara-Dex: ixazomib-daratumumab-dexamethasone; Dara-RD: daratumumab-lenalidomide-dexamethasone. For other studies in MM see also Mina et al.51
1184
haematologica | 2020; 105(5)