Page 199 - Haematologica May 2020
P. 199
miR-146b in T-LGL Leukemia patients
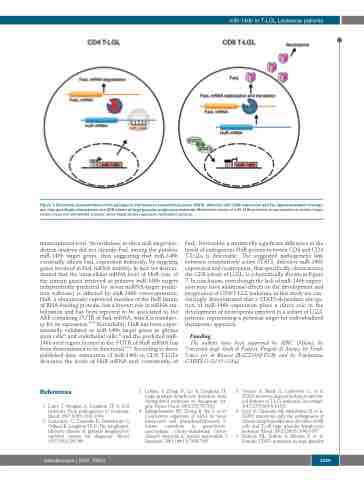
Figure 7. Schematic representation of the pathogenic link between constitutively active STAT3, defective miR-146b expression and Fas ligand-mediated neutrope- nia, that specifically characterizes the CD8 subset of large granular lymphocyte leukemia. Methylation status of miR-146b promoter is represented as circles: empty circles mean not methylated cytosine, while black circles represent methylated cytosine.
transcriptional level. Nevertheless, in silico miR-target pre- diction analysis did not identify FasL among the putative miR-146b target genes, thus suggesting that miR-146b eventually affects FasL expression indirectly, by targeting genes involved in FasL mRNA stability. In fact, we demon- strated that the intracellular mRNA level of HuR (one of the sixteen genes retrieved as putative miR-146b targets independently predicted by seven miRNA-target predic- tion software) is affected by miR-146b over-expression. HuR, a ubiquitously expressed member of the HuR family of RNA-binding proteins, has a known role in mRNA sta- bilization and has been reported to be associated to the ARE-containing 3’UTR of FasL mRNA, which is mandato- ry for its expression.29,36 Remarkably, HuR has been exper- imentally validated as miR-146b target genes in glioma stem cells31 and endothelial cells,30 and the predicted miR- 146b seed region located in the 3’UTR of HuR mRNA has been demonstrated to be functional.30,31 According to these published data, restoration of miR-146b in CD8 T-LGLs decreases the levels of HuR mRNA and, consistently, of
FasL. Noticeably, a statistically significant difference in the levels of endogenous HuR protein between CD8 and CD4 T-LGLs is detectable. The suggested pathogenetic link between constitutively active STAT3, defective miR-146b expression and neutropenia, that specifically characterizes the CD8 subset of LGLL, is schematically shown in Figure 7. In conclusion, even though the lack of miR-146b expres- sion may have additional effects on the development and progression of CD8 T-LGL leukemia, in this study we con- vincingly demonstrated that a STAT3-dependent abroga- tion of miR-146b expression plays a direct role in the development of neutropenia reported in a subset of LGLL patients, representing a potential target for individualized therapeutic approach.
Funding
The authors have been supported by AIRC (Milan), by Università degli Studi di Padova, Progetti di Ateneo, by Fondo Unico per la Ricerca (BAZZONI-FUR) and by Fondazione CARIPLO (2015-0584).
References
1. Lamy T, Moignet A, Loughran TP, Jr. LGL leukemia: from pathogenesis to treatment. Blood. 2017;129(9):1082-1094.
2. Semenzato G, Zambello R, Starkebaum G, Oshimi K, Loughran TP, Jr. The lymphopro- liferative disease of granular lymphocytes: updated criteria for diagnosis. Blood. 1997;89(1):256-260.
3. Leblanc F, Zhang D, Liu X, Loughran TP. Large granular lymphocyte leukemia: from dysregulated pathways to therapeutic tar- gets. Future Oncol. 2012;8(7):787-801.
4. Epling-Burnette PK, Zhong B, Bai F, et al. Cooperative regulation of Mcl-1 by Janus kinase/stat and phosphatidylinositol 3- kinase contribute to granulocyte- macrophage colony-stimulating factor- delayed apoptosis in human neutrophils. J Immunol. 2001;166(12):7486-7495.
5. Teramo A, Barila G, Calabretto G, et al. STAT3 mutation impacts biological and clin- ical features of T-LGL leukemia. Oncotarget. 2017;8(37):61876-61889.
6. Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120(15):3048-3057.
7. Koskela HL, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular
haematologica | 2020; 105(5)
1359