Page 313 - Haematologica April 2020
P. 313
HSC-GT for herPAP
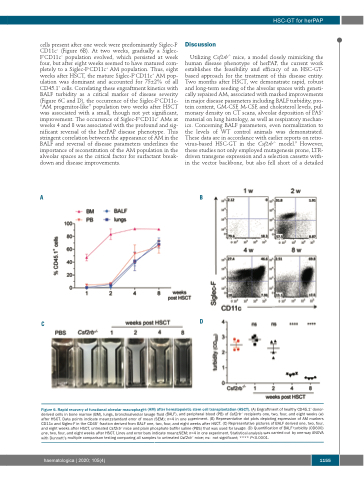
cells present after one week were predominantly Siglec-F- CD11c− (Figure 6B). At two weeks, gradually a Siglec- F+CD11c− population evolved, which persisted at week four, but after eight weeks seemed to have matured com- pletely to a Siglec-F+CD11c+ AM population. Thus, eight weeks after HSCT, the mature Siglec-F+CD11c+ AM pop- ulation was dominant and accounted for 75±2% of all CD45.1+ cells. Correlating these engraftment kinetics with BALF turbidity as a critical marker of disease severity (Figure 6C and D), the occurrence of the Siglec-F+CD11c- “AM progenitor-like” population two weeks after HSCT was associated with a small, though not yet significant, improvement. The occurrence of Siglec-F+CD11c+ AMs at weeks 4 and 8 was associated with the profound and sig- nificant reversal of the herPAP disease phenotype. This stringent correlation between the appearance of AM in the BALF and reversal of disease parameters underlines the importance of reconstitution of the AM population in the alveolar spaces as the critical factor for surfactant break- down and disease improvements.
Discussion
Utilizing Csf2rb-/- mice, a model closely mimicking the human disease phenotype of herPAP, the current work establishes the feasibility and efficacy of an HSC-GT- based approach for the treatment of this disease entity. Two months after HSCT, we demonstrate rapid, robust and long-term seeding of the alveolar spaces with geneti- cally repaired AM, associated with marked improvements in major disease parameters including BALF turbidity, pro- tein content, GM-CSF, M-CSF, and cholesterol levels, pul- monary density on CT scans, alveolar deposition of PAS+ material on lung histology, as well as respiratory mechan- ics. Concerning BALF parameters, even normalization to the levels of WT control animals was demonstrated. These data are in accordance with earlier reports on retro- virus-based HSC-GT in the Csf2rb-/- model.9 However, these studies not only employed mutagenesis prone, LTR- driven transgene expression and a selection cassette with- in the vector backbone, but also fell short of a detailed
AB
C
D
Figure 6. Rapid recovery of functional alveolar macrophages (AM) after hematopoietic stem cell transplantation (HSCT). (A) Engraftment of healthy CD45.1+ donor- derived cells in bone marrow (BM), lungs, bronchoalveolar lavage fluid (BALF), and peripheral blood (PB) of Csf2rb-/- recipients one, two, four, and eight weeks (w) after HSCT. Data points indicate mean±standard error of mean (SEM); n=4 in one experiment. (B) Representative dot plots depicting expression of AM markers CD11c and Siglec-F in the CD45+ fraction derived from BALF one, two, four, and eight weeks after HSCT. (C) Representative pictures of BALF derived one, two, four, and eight weeks after HSCT, untreated Csf2rb-/- mice and plain phosphate buffer saline (PBS) that was used for lavage. (D) Quantification of BALF turbidity (OD600) one, two, four, and eight weeks after HSCT. Lines and error bars indicate mean±SEM; n=4 in one experiment. Statistical analysis was carried out by one-way ANOVA with Dunnett’s multiple comparison testing comparing all samples to untreated Csf2rb-/- mice; ns: not significant; **** P<0.0001.
haematologica | 2020; 105(4)
1155