Page 244 - Haematologica April 2020
P. 244
D.E. van der Wal et al.
acid, increased membrane association of NEU1 (Figure 2D) and was even more pronounced for NEU2 (Figure 2E). Although collagen binds to VWF upon vascular injury, it did not induce NEU membrane translocation. AA also sig- nificantly increased NEU1, which is in line with earlier findings that AA induced clustering of GPIbα, which may lead to subsequent desialylation (Figure 2D).32 The VWF- induced increase in membrane NEU was less pronounced in PRP (Figure 2A-B) when compared to washed platelets (Figure 2D-E), suggesting a potential inhibitory effect of plasma proteins on NEU activation. Risto-only controls (without VWF-addition) did not induce NEU membrane expression (Online Supplementary Figure S1C). GPIbα is also clustered by low concentrations of thrombin,33 how- ever thrombin-stimulated washed platelets were not desialylated (Online Supplementary Figure S1D). Binding of all lectins was similar to unstimulated controls; demon- strating desialylation is specific for the VWF-GPIbα inter- action. This data demonstrate that desialylation occurs upon specific clustering of GPIbα through binding to its ligand VWF, which in turn triggers membrane association of NEU1 and NEU2.
Signalling pathways involved in NEU expression
which might potentiate α-/δ-granule/lysosome-release, these were examined following VWF/risto-stimulation. Both P-selectin and LAMP-1 surface expression following VWF/risto-stimulation were increased as a consequence of the longer incubation times (Online Supplementary Figure
A
B
C
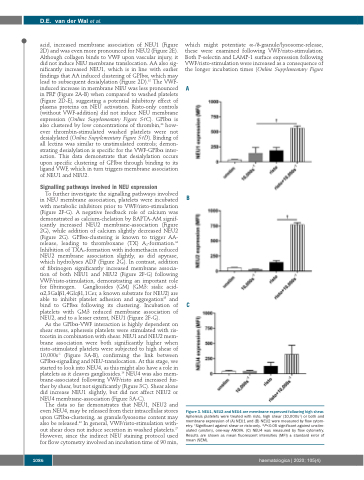
Figure 3. NEU1, NEU2 and NEU4 are membrane expressed following high shear.
Apheresis platelets were treated with risto, high shear (10,000s-1) or both and
To further investigate the signalling pathways involved in NEU membrane association, platelets were incubated with metabolic inhibitors prior to VWF/risto-stimulation (Figure 2F-G). A negative feedback role of calcium was demonstrated as calcium-chelation by BAPTA-AM signif- icantly increased NEU2 membrane-association (Figure 2G), while addition of calcium slightly decreased NEU2 (Figure 2G). GPIbα-clustering is known to trigger AA- release, leading to thromboxane (TX) A2-formation.34 Inhibition of TXA2-formation with indomethacin reduced NEU2 membrane association slightly, as did apyrase, which hydrolyses ADP (Figure 2G). In contrast, addition of fibrinogen significantly increased membrane associa- tion of both NEU1 and NEU2 (Figure 2F-G) following VWF/risto-stimulation, demonstrating an important role for fibrinogen. Gangliosides (GM) (GM3: sialic acid- α2,3Galβ1,4Glcβ1,1Cer, a known substrate for NEU2) are able to inhibit platelet adhesion and aggregation35 and bind to GPIbα following its clustering. Incubation of platelets with GM3 reduced membrane association of NEU2, and to a lesser extent, NEU1 (Figure 2F-G).
As the GPIbα-VWF interaction is highly dependent on shear stress, apheresis platelets were stimulated with ris- tocetin in combination with shear. NEU1 and NEU2 mem- brane association were both significantly higher when risto-stimulated platelets were subjected to high shear of 10,000s-1 (Figure 3A-B), confirming the link between GPIbα-signalling and NEU-translocation. At this stage, we started to look into NEU4, as this might also have a role in platelets as it cleaves gangliosides.16 NEU4 was also mem- brane-associated following VWF/risto and increased fur- ther by shear, but not significantly (Figure 3C). Shear alone did increase NEU1 slightly, but did not affect NEU2 or NEU4 membrane-association (Figure 3A-C).
The data so far demonstrates that NEU1, NEU2 and even NEU4, may be released from their intracellular stores upon GPIbα-clustering, as granule/lysosome content may also be released.36 In general, VWF/risto-stimulation with- out shear does not induce secretion in washed platelets.27 However, since the indirect NEU staining protocol used for flow cytometry involved an incubation time of 90 min,
membrane expression of (A) NEU1 and (B) NEU2 were measured by flow cytom- †
etry. Significant against shear or risto-only. *P<0.05 significant against unstim- ulated (unstim), one-way ANOVA. (C) NEU4 was measured by flow cytometry. Results are shown as mean fluorescent intensities (MFI) ± standard error of mean (SEM).
1086
haematologica | 2020; 105(4)