Page 329 - Haematologica March 2020
P. 329
Prevention of VTE in cancer patients
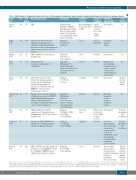
Table 2. Main features of randomized studies on the role of anticoagulants in ambulatory cancer patients receiving chemotherapy with death as the primary outcome.
Author, D-B N. of year patients
Labeau, No 277 199430
Kakkar, Yes 374 200431
Main inclusion criteria
SCLC
Advanced stage III or IV (locally advanced or metastatic) cancer*
of the breast, lung, gastrointestinal tract, pancreas, liver, genitourinary tract, ovary, or uterus; age between 18 and
80 years.
SCLC* , ECOG PS < 3; age between 18 and 75 years.
Adult patients with metastatic or locally advanced solid tumors*
Advanced breast cancer failed first-line chemotherapy, advanced prostate cancer failed primary hormonal therapy, advanced
lung cancer, or advanced colorectal. ECOG PS <2, life expectancy
>12 weeks, age >18 years
Prostate cancer* <6 m after diagnosis of hormone-refractory state, NSCLC*
without clinically significant pleural effusion <3 m after diagnosis of
stage IIIB, or locally advanced pancreatic cancer* <3 m after diagnosis
FIGO stage IIB to IV epithelial
ovarian cancer*, primary peritoneal
or Fallopiantube cancer*; age between 18 and 75 years
Limited stage SCLC*, ECOG PS ≤2, platelets >100,000/mm3 and absence of active bleeding; age >18 years.
SCLC or NSCLC* <6 weeks of diagnosis; age 18 years or older; ECOG-PS 0-3; able to self-administer LMWH or have it administered to them by a caregiver.
Experimental
Heparin calcium
(initially 500 IU/kg/day then adjusted by clotting times 2-3 times normal value) t.d. (70 patients)
or t.i.d. (62 patients) times daily vs. No heparin
Dalteparin (5,000 IU o.d.) vs. Placebo
Dalteparin, (5,000 IU o.d.) vs. No dalteparin
Nadroparin
(BW adjusted**,
t.d. during the initial
14 days and o.d. thereafter for another 4 weeks)
vs Placebo.
Dalteparin (5,000IU o.d.) vs. Placebo
Nadroparin (BW-adjusted therapeutic
dose for 2 weeks,
and 4 weeks at half therapeutic dose)
vs. No nadroparin Dalteparin
(50 IU/kg, 100 IU/kg, or 150 IU/kg)
vs. No dalteparin
bemiparin (3,500 IU/day) vs. No bemiparin
Deltaparin (5,000 IU/day) vs. No dalteparin
Duration of follow-up
from randomization to the sixth course of CHT
1 year
1 year
6 weeks
13 months
46 weeks
Six 21-day cycles
12 months
1 year
Duration of prophylaxis
5 weeks (with 1week
stop after the second course
of CHT)
1 year or until death
18 weeks
6 weeks
2 years
46 weeks
Definition of major bleeding
Not reported
According to standard criteria39
Not specified
Clinically overt associated with hemoglobin decrease >2 g/dL, requiring
>2 transfusion, or retroperitoneal or intracranial.
Not specified
Overt with hemoglobin decrease >2 g/dL or
transfusion >2 units.
Study completed
Yes
Yes
Yes
Yes
Stopped after first interim analysis
Yes
Premature interruption slow recruitment
Altinbas, No 200432
84
Klerk, Yes 302 200533
Sideras, Yes 138 200634
van Doormaal, 201135
Elit, 201236
Lecumberri, 201337
Macbeth, 201538
No
No
No
No
503
86
38
2202
Within7days Notspecified prior to the
cycle 1 of CHT
to day 21
of cycle 3
26 weeks
24 weeks
Associated Premature
with hemoglobin decrease >2 g/dL,
or transfusion
>2 units, involved
a critical site, contributed to death, or any clinically relevant bleeding requiring the
stop of treatment
interruption slow recruitment
Associated with
death, occurred
at a critical site or resulted in transfusion >2 units, or hemoglobin
decrease >2.0 g/dL
The trial did not reach the intended number of outcome events
SCLC: small cell lung cancer; IU: International Units; t.d.: twice daily; t.i.d.:.ter in die; CHT: chemotherapy; o.d.: once daily; ECOG: Eastern Cooperative Oncology Group; PS: Performance Status; BW: body weight; NSCLC: non-small cell lung cancer; FIGO: International Federation of Gynecology and Obstetrics; LMWH: low molecular weight heparin. * histologically confirmed; **0.4 mL if body weight <50 kg, 0.6 mL if body weight between 50 and 70 kg, and 0.8 mL if body weight > 70 kg. ° defined according to GCIG-CA125 response criteria.
haematologica | 2020; 105(3)
843