Page 26 - Haematologica March 2020
P. 26
S.L. Thein et al.
risk of alloimmunization in SCD is increased when patients are transfused for acute complications, such as acute chest syndrome, acute pain and acute multi-system organ failure, which are clinical complications marked by significant inflammation. Thus, unique aspects of transfu- sion therapy in patients with SCD, in conjunction with other possible immune perturbations, appear to place such patients at a particular risk of RBC alloimmunization.5,14-17
Definitions of acute and delayed hemolytic transfusion reactions and hyperhemolysis
While acute hemolytic transfusion reactions can largely be avoided by stringent alloantibody investigations prior to transfusion, DHTR, which typically occur days or weeks following the implicated transfusion episode of
A
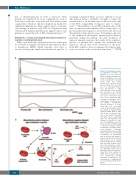
seemingly compatible RBC,7 are more difficult to avoid. The delayed nature of DHTR is thought to reflect the recrudescence of an alloantibody not detected at the time of the RBC compatibility testing just prior to transfu- sion.6,18,19 The inability to detect RBC alloantibodies at the time of transfusion presumably reflects evanescence of a prior alloantibody response to a level below the detection threshold in routine clinical assays. Following re-exposure to the implicated alloantigen, immunological memory generated during the primary encounter facilitates an amnestic immune response that results in the rapid pro- duction of alloantibodies against the transfused unit (Figure 1). This in turn causes destruction of the trans- fused RBC, which is often accompanied by clinical symp- toms associated with accelerated hemolysis.18,19 DHTR
Figure 1. Delayed-type hemolyt- ic transfusion reactions. (A) Exposure to a red blood cell (RBC) alloantigen through trans- fusion or pregnancy can result in the development of alloanti- bodies (allo) that quickly evanescence over time, possi- bly preventing their detection prior to a subsequent transfu- sion. Re-exposure to RBC expressing the same alloanti- gen can induce an amnestic alloantibody response, which can cause accelerated clear- ance of the transfused RBC, resulting in hemolysis and a delayed-type transfusion reac- tion (DHTR). Alloantibody- induced clearance of trans- fused RBC can occasionally result in hyperhemolysis, other- wise known as hyperhemolytic syndrome (HHS), which is signi- fied by the accelerated clear- ance of the patient’s own RBC and which can be particularly fatal. (B). Alloantibodies that develop in response to expo- sure to alloantigens can lead to direct clearance of RBC through a variety of antibody effector mechanisms, including comple- ment activation. Sometimes patients will experience a DHTR in the absence of a detectable alloantibody; an alloantibody may be present and simply be below the detection threshold of clinical assays or an alloanti- body may be absent entirely, with the DHTR possibly reflect- ing heme-mediated comple- ment activation and RBC hemol- ysis. Regardless of the mode of hemolysis experienced by trans- fused RBC in the setting of a DHTR, heme released may acti- vate complement, thereby potentially contributing to the development of hyperhemoly- sis. Trx: transfusion; Hb: hemo- globin.
B
540
haematologica | 2020; 105(3)