Page 58 - 2020_02-Haematologica-web
P. 58
R. Vilar et al.
degree of obesity.121,122 Thus, obesity represents a major
risk factor for other pathologies, including thromboembol-
ic events, CVD, diabetes, cancer and fatty liver dis- ease.120,121
Fibrinogen may be involved in the pathology of obesity. Levels of fibrinogen are higher in obese patients with type 2 diabetes than in obese subjects without type 2 dia- betes.123 In addition, plasma fibrinogen levels correlate with fasting insulin levels and disease state advancement in noninsulin-dependent diabetics,124 and while insulin infusion decreases fibrinogen biosynthesis in normal sub- jects,125 insulin resistance/deficiency may contribute to hyperfibrinogenemia.126 Furthermore, fibrinogen from dia- betic patients generates denser, fibrinolysis-resistant clots, while insulin treatment leads to changes in fibrinogen and a more permeable clot.127 These alterations have been attributed to the glycation of fibrinogen and its effect on fibrin clots, potentially contributing to the risk of throm- bosis.
Mice fed with a high-fat diet developed fibrin(ogen) deposits in white adipose tissue and liver which co-local- ized with macrophage accumulation.128 In contrast to Fibγ∆5 mice and mice without factor XIIIA, Fibγ390–396A animals were protected from increased body weight with a high- fat diet, specifically at the fat mass level. These animals, with fibrin(ogen)-γ residues 390 to 396 replaced with ala- nine and therefore lacking an αMβ2-binding motif on fib- rin(ogen),129 showed less systemic and local inflammation, demonstrated by lower levels of pro-inflammatory mole- cules, adipose tissue macrophages and smaller white adi- pose tissue adipocytes128 when compared to those of wild- type animals. In addition, the fibrinogen γ390–396A vari- ant led to lower liver weight, steatosis, serum alanine aminotransferase and hepatic inflammatory markers, and conferred some degree of protection against the develop-
ment of induced fatty liver disease. Glucose clearance and insulin sensitivity were improved, revealing improved glu- cose metabolism.
Thus fibrin(ogen)-driven inflammation, via leukocyte interactions in adipose tissue and liver, worsens obesity and increases its downstream harmful effects. Targeting thrombin or fibrin(ogen) may improve the morbidity of obesity-linked pathologies.
Amyloidosis
Amyloidosis is a group of disorders originating from mutations that cause conformational changes, typically involving β-sheet structures, in soluble proteins. These then aggregate as extracellular amyloid fibril deposits in various organs. In systemic forms, amyloidosis can pro- gressively induce organ dysfunction, and be fatal.130
Fibrinogen-driven renal hereditary amyloidosis is a rare group of disorders with autosomal-dominant inheritance caused by heterozygosity for mutations in the αC- domain, which result in improper folding and amyloid for- mation followed by accumulation and deposition in the kidneys.66 Other elements present in the deposits can fur- ther contribute to fibrin(ogen) amyloid formation, namely amyloidosis-enhancing factor131 and serum amyloid A.132 The latter binds to purified fibrinogen and induces amy- loid formation and spontaneous dense, matted fibrin(ogen) deposits, independently of thrombin. However, its involvement in renal amyloidosis has not yet been demonstrated.
Fibrinogen-derived amyloid deposits disrupt kidney structure and impair kidney function, effects that become more severe over time, with accumulation of the amyloid. Amyloidosis is associated with hypertension, nephrotic
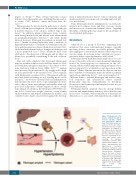
Figure 4. Pathogenesis of fib- rinogen amyloidosis and its visceral, vascular, cardiac and neurological implica- tions. Fibrinogen variants are produced in the liver where they may rarely cause hepatic amyloidosis. In addition to the kidney, where deposits prompt renal failure, fibrino- gen has different targets. It may also accumulate in vas- cular and cardiac walls, resulting in impaired endothelial function. This, together with nephrotic syn- drome, hyperlipidemia and hypertension, facilitates atheroma formation and eventually results in coronary atherosclerosis. Fibrinogen may be the basis of neuro- pathic features as well as the symptoms of gut dysmotility in patients with fibrinogen amyloidosis. This image was adapted from Picken MM135 and created with BioRender.com.
292
haematologica | 2020; 105(2)