Page 28 - 2020_02-Haematologica-web
P. 28
C. Camaschella et al.
Erythroblasts may also export heme through feline leukemia virus C receptor (FLVCR).31,32 The latter two export mechanisms seem counterintuitive in cells that need iron/heme for the production of hemoglobin, but are likely biological safeguard mechanisms that protect erythroid cells from iron/heme excess.
Iron homeostasis
Maintaining iron balance requires tight regulation at cel- lular, systemic and tissue levels.
Cell iron homeostasis
The IRP-IRE system
This system is based on the post-transcriptional control of iron genes mediated by the interaction of IRP with IRE of their mRNA untranslated regions. In iron-deficiency states, IRP1 and 2 increase iron uptake by stabilizing TFR1 mRNA and blocking iron storage and export by suppress- ing ferritin and ferroportin translation. In iron-replete cells, Fe/S clusters convert IRP1 into cytosolic aconitase, while IRP2 undergoes iron-dependent proteasomal degradation. The IRP1/aconitase interconversion on the one hand links iron to tricarboxylic acid and cell metabolism, and on the
B
A
AB
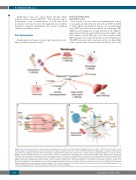
Figure 1. The iron cycle. Iron (Fe) circulates bound to transferrin to be released to all organs/tissues through transferrin receptor 1. Most iron (20-25 mg) recycled by macrophages, which phagocytize senescent red blood cells (RBC), is supplied to the bone marrow for RBC production. The daily uptake of dietary iron by duodenal enterocytes is 1-2 mg; the same amount is lost through cell desquamation and blood loss. Excess iron is stored in the liver and macrophages as a reserve. Arrows indicate directions. Numbers (in mg) are a mean estimate. (A) Focus on intestinal iron absorption. The metal transporter DMT1 takes up ferrous iron, reduced by DCYTB, on the luminal side of the enterocyte. Iron not used inside the cell is either stored in ferritin (FT) or exported to circulating transferrin (TF) by ferroportin (FPN), after ferrous iron is oxidized to ferric iron by hephaestin (HEPH).1 Hypoxia inducible factor (HIF)-2α, stabilized by local hypoxia, stimulates the expression of the apical (DMT1) and basolateral (FPN) transporters.63 Heme, after entering the cell through an unknown mechanism, is converted to iron by heme oxygenase. (B) Focus on the iron recycling process. Macrophages recover iron from phagocytized RBC after heme is degraded by heme oxygenase. They also recover heme from hemoglobin (Hb)-haptoglobin (HP) or heme-hemopexin (HPX) complexes.2 Iron not used inside the cells is either stored in FT or exported to the circulation by FPN with the coop- eration of ceruloplasmin (CP). The latter is the preferential route in normal conditions.
262
haematologica | 2020; 105(2)