Page 40 - 2019_11 Resto del Mondo-web
P. 40
M. Fürstenau et al.
above-mentioned phase II trial in high-risk CLL was only 6%.16,19 Atrial fibrillation has been reported in several trials as a cause of treatment interruption. In a retrospective analysis, the 5-year incidence of atrial fibrillation was 21%.16 Another pooled analysis of multiple clinical trials estimated a 3-year cumulative incidence of atrial fibrilla- tion of 13.8% among ibrutinib-treated patients.20
Other BTK inhibitors have been developed to overcome these commonly encountered difficulties, such as the development of resistance mutations and the discontinua- tion of treatment due to adverse drug effects. While sec- ond-generation inhibitors such as substance ARQ 531 promise efficacy in the context of BTK C481S mutations, more specific inhibitors, including acalabrutinib and zanubrutinib, appear to cause fewer adverse off-target effects.21-24 Direct, randomized comparisons of acalabruti- nib (NCT02477696) and zanubrutinib (NCT03734016) against ibrutinib are currently ongoing.
and/or TP53 mutations for whom no other therapies are appropriate. Idelalisib has shown some activity as a single agent in r/r CLL and was combined with rituximab in a prospective randomized study against rituximab monotherapy.4,25 The median PFS in the placebo group was 5.5 months and was not reached in the rituximab-ide- lalisib arm [hazard ratio (HR) for disease progression or death 0.15, P<0.001]; the ORR was 81% with idelalisib, but only 13% in the rituximab arm. Following these encouraging results, the combination was investigated in the first-line setting. In a phase II study, 64 patients were treated with rituximab and idelalisib with a median treat- ment duration of 22.4 months.26 The ORR was 97%, including 19% complete responses (CR) and the estimated 3-year PFS was 83%. However, significant severe adverse events of this regimen were reported. Diarrhea and colitis occurred in 61% of patients, skin rash in 58%, fever in 42%, nausea in 38% and transaminitis in 67%.27 In large phase III trials, an increased mortality was observed in the idelalisib-containing arms which led to premature discon- tinuation of other trials and a re-evaluation of the sub- stance by regulatory authorities.28
Other kinase inhibitors targeting the PI3K pathway are umbralisib and the dual PI3K inhibitor duvelisib. While umbralisisb treatment is not yet approved, the use of duvelisib has been approved by the Food and Drug
PI3K inhibitors
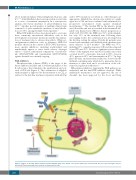
Phosphoinositide 3-kinase (PI3K)γ is the target of the kinase inhibitor idelalisib and a downstream kinase of the B-cell receptor that stimulates the proliferation and sur- vival of CLL cells (Figure 1). The combination of idelalisib with rituximab is approved for the treatment of r/r CLL as well as for the first-line treatment of patients with del(17p)
Figure 1. Targets of currently approved (black) and investigated (gray) novel agents. CLL: chronic lymphocytic leukemia; BCR: B-cell receptor. This figure was pro- duced by M. Fürstenau using servier medical art (smart.servier.com).
2146
haematologica | 2019; 104(11)