Page 20 - 2019_11 Resto del Mondo-web
P. 20
2126
Editorials
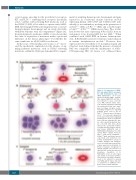
occur in genes encoding for the growth factor receptors KIT and FLT3,20,21 rendering these receptors chronically active, together with activating RAS mutations such as the K-RAS (G12D), all of which co-operate with AML1- ETO. Interestingly, K-RAS activating mutations occur late during leukemia development and are rarely detectable within the leukemic stem cell compartment21 (Figure 1A). In myelodysplastic syndromes (MDS), it was shown that the order of acquisition of mutations makes a profound difference on the disease phenotype,22 but whether the same stands true for t(8;21) AML is not known.
In this issue of Haematologica, Di Genua et al.23 uncov- ered the mechanistic explanation for the absence of sig- naling pathway mutations, such as K-RAS activating mutations, within the t(8;21) pre-leukemic HSC compart-
ment by analyzing hematopoietic development and gene expression in conditional murine knock-in models expressing human AML1-ETO and K-RAS(G12D) indi- vidually or in combination, resulting in the generation of an Aml1ETO/+ (AM), a K-RasG12D/+ (KM), and a double-target- ed Aml1ETO/+; K-RasG12D/+ (AKM) mouse lines. Prior studies had shown that mice expressing K-Ras(G12D) from its endogenous locus develop MDS but not AML.24 When combined with AML1-ETO in human hematopoietic cells, K-RAS(G12D) promoted leukemic transformation in murine transplantation models.25 However, here expression levels were likely to be non-physiological and it had not been defined whether the presence of mutated RAS was compatible with the maintenance of AML1- ETO-expressing HSC. Di Genua et al. addressed these
Figure 1. Co-expression of AML1- ETO and mutant RAS is incompati- ble with hematopoietic stem cell (HSC) maintenance. (A) In acute myeloid leukemia (AML) patients, HSC express AML1-ETO, but this is not sufficient to cause overt dis- ease. AML leukemic blasts repre- sent myeloid progenitors harboring the translocation that have under- gone secondary oncogenic events, including mutations in signaling pathway genes such as the K- RASG12D/+. However, prior to Di Genua et al.,23 the molecular explanation for the lack of mutations in signaling pathway genes in the pre-leukemic stem cells was unknown. HSC (pur- ple cells), myeloid progenitors (pur- ple cells with pink dots). (B) Di Genua et al.23 generated condition- al murine knock-in models express- ing human AML1-ETO and K- RAS(G12D) individually or in combi- nation: Aml1ETO/+ (purple nuclei), K- RasG12D/+ (pink nuclei), and Aml1ETO/+ K-RasG12D/+(pink and dotted nuclei), respectively, and performed com- petitive transplantation assays to compare wild-type and transgenic stem cell activity and measure glob- al gene expression as indicated. (C) Model of step-wise oncogenesis in t(8;21) patients: the presence of t(8;21) in HSC results in a quiescent phenotype. Acquisition of K- RAS(G12D) occurs at later stages and results in increased prolifera- tion. However, the double oncogenic event is not sufficient to develop overt AML, and the nature and order of acquisition of additional onco- genic events remains unknown.
haematologica | 2019; 104(11)