Page 188 - 2019_11 Resto del Mondo-web
P. 188
J. Series et al.
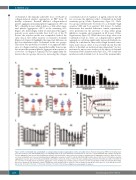
of ibrutinib at the clinically achievable dose of 0.5 mM on collagen-induced platelet aggregation in PRP from 70 healthy volunteers. Ibrutinib inhibited collagen-induced platelet aggregation (maximal platelet aggregation <50%) in 56% of healthy donors while it had no or little effect (max- imal platelet aggregation >50%) in the remaining 44% (Figure 1A). Interestingly, when we performed this aggre- gometry assay again 6 months later in 29 out of the 70 donors, the response profile was comparable. Indeed, the same donors were either sensitive or resistant to ibrutinib (Figure 1A). Figure 1B highlights the important difference in the dose-dependent effect of ibrutinib in the two groups. This effect was probably not related to an apparent differ- ence of collagen sensitivity among the healthy donor popu- lation since the maximal platelet aggregation in response to a low dose of collagen (3.3 mg/mL) was not significantly dif- ferent in the two groups. Moreover, increasing the collagen
concentration from 3.3 μg/mL to 6 μg/mL reduced but did not overcome the inhibitory effect of ibrutinib in the high sensitivity group (Online Supplementary Figure S1). These two groups will hereafter be referred to as ibrutinib “high sensitive” (HS) and “low sensitive” (LS) donors. To further characterize this marked difference, similar experiments were performed in the presence of drug efflux pump inhibitors, reserpine and verapamil, in LS donors (Online Supplementary Figure S2). While these two drugs alone or in combination had no effect on collagen-induced platelet aggregation, each drug significantly increased ibrutinib sen- sitivity. In combination, they induced high ibrutinib sensi- tivity in LS donors. Since it was recently shown that the effects of ibrutinib are incubation time-dependent,27 we also performed a time-course analysis of the effects of ibrutinib treatment (Online Supplementary Figure S2C). We found that incubation with ibrutinib for 1 h caused the maximal inhi-
AB
CD
E
Figure 1. Effect of ibrutinib and acalabrutinib on collagen-induced platelet aggregation in vitro, in healthy donors. Platelet-rich plasma (PRP) from healthy volun- teers was treated or not with ibrutinib (A-D) or acalabrutinib (ACP) (C-E) at the indicated concentrations for 1 h at 37°C and stimulated with collagen 3.3 mg/mL. Platelet aggregation was assessed by turbidimetry during 10 min and results, expressed as percentage of maximal platelet aggregation and area under the curve, are mean ± standard error of mean. A maximal platelet aggregation response below 50% indicated ibrutinib high-sensitive donors (HS, in red, n=39) while a maximal aggregation response above 50% indicated ibrutinib low-sensitive donors (LS, in blue, n=31). The same analysis was performed 6 months later (After 6 months) in 29 out of the 70 healthy donors (A). Platelet aggregation curves showing representative platelet responses to ACP and ibrutinib on PRP from ibrutinib HS healthy donors are shown (D). The numbers of donors analyzed in each experiment were: (A) n=70, after 6 months: n=29; (B) LS: n=15, HS: n=7; (C) n=52; (E) LS: n=10, HS: n= 12. *P<0.05, **P<0.01, ***P<0.001, #P<0.05, ##P<0.01, ###P<0.001 according to one-way analysis of variance.
2294
haematologica | 2019; 104(11)