Page 12 - 2019_11 Resto del Mondo-web
P. 12
Editorials
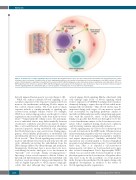
Figure 1. Potential routes of Notch signaling in the bone marrow. Notch ligands and receptors are expressed by both non-hematopoietic and hematopoietic cellular elements in the bone marrow. Potential routes of Notch signaling that influence the function of hematopoietic stem and progenitor cells (HSPC) include: ( ) inter- action of Notch ligands in endothelial cells with Notch receptors in HSPC; ( ) Notch ligand-receptor interactions between endothelial cells, with indirect effects on HSPC; ( ) interaction of Notch receptors in HSPC with Notch ligands expressed by other hematopoietic cells. In this issue, Shao et al. provide new data in support of Notch signaling within the endothelium ( ) as a critical regulator of hematopoietic recovery after bone marrow injury.
lial cells impaired hematopoietic recovery (Figure 1, ). While the authors identified Notch signaling as an essential component of the response to injury in the bone marrow, the mechanisms underlying Notch’s impact in this context remain unclear. The bone marrow injury response includes a complex interplay of signaling cues secreted from multiple cellular sources. For example, VEGF-A, as well as Angiopoietin-1, are thought to control regeneration and reassembly of the bone marrow vascu- lature.15,16 Importantly, the cellular source, role and regula- tion of individual factors may differ markedly between steady-state conditions and after bone marrow injury.18 Shao et al. found that lack of Notch activation after injury increased apoptosis among endothelial cells, suggesting that Notch functions as a pro-survival cue. During angio- genesis, Notch inhibits proliferation of endothelial cells and ultimately allows for proper formation of functional blood vessels.19 Thus, Notch signaling may restrict bone marrow endothelial cell activation and entry into the cell cycle, ultimately protecting the endothelium from the DNA damage induced by chemotherapy and irradiation. Alternatively, Notch may have a more direct role in re- establishing the niche, analogous to its involvement in “tip/stalk” cell crosstalk during neoangiogenesis.19 Sprouting of new vessels requires a delicate balance of tip/stalk cell differentiation in which tip endothelial cells lead new vessel sprouting for invasion and migration. Tip cells are highly responsive to VEGF, require a high gly- colytic flux and, although they express Dll4, do not
actively engage Notch signaling. On the other hand, stalk cells undergo high levels of Notch signaling which reduces expression of VEGFR2/3 and glycolytic enzymes, ultimately helping to repress the tip cell fate while main- taining stalk cell identity.19 Thus, Notch activity may be important during early stages of bone marrow vascula- ture reassembly by regulating pathways similar to tip/stalk cell differentiation and by integrating angiogenic cues with the metabolic status of the endothelium. Finally, it is possible that Notch acts through yet to be dis- covered mechanisms unique to the bone marrow vascu- lature, whose regulation during steady-state conditions and after injury remains only partially understood.
Notch signaling may also have roles in hematopoiesis beyond its functions in the HSPC niche. Dll4 inactivation in mesenchymal progenitor cells was reported to decrease bone marrow common lymphoid progenitor numbers and impair thymopoiesis.12 Similarly, endothelial Dll4 inactivation was recently linked to decreased lymphoid progenitors and enhanced myelopoiesis.5 Consistent with these data, Shao et al. reported a cell-autonomous hematopoietic cell defect in T-cell production by mice that received transplanted Notch1 hypomorphic HSPC, which was associated with decreased numbers of lym- phoid progenitors in the bone marrow. Altogether, these data leave room for the possibility of a bone marrow niche that provides prethymic Notch signals during early lymphoid development, in addition to the effects of Notch signaling in niche regeneration.
2118
haematologica | 2019; 104(11)