Page 21 - 2019_10 resto del Mondo_web
P. 21
Editorials
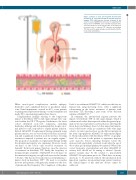
Figure 1. Impact of acute and long-term microvascular thrombosis in congenital thrombotic thrombocytopenic purpura (TTP). Microvascular thrombi formation is the result of severe ADAMTS 13 deficiency in congenital TTP. Replacement of ADAMTS 13 is currently via plasma infu- sion. Presentation of acute TTP episodes, or the impact of recurrent acute or subacute TTP episodes in hereditary TTP, may result in organ damage, specifically affecting the heart, brain and kidneys.
Other neurological complications include epilepsy, headaches, and a significant history of psychiatric symp- toms. Renal impairment occurred in 26%, some patients requiring renal replacement therapy or renal transplant and nearly 50% experiencing jaundice/liver disease.
Complimentary findings relating to the longer-term impact of hereditary TTP on end-organ damage were cap- tured within the UK TTP registry. Furthermore, the latter cohort identified non-overt symptoms, including headaches, lethargy, and abdominal pain that, despite a nor- mal platelet count, responded to regular plasma infusion. Indeed, ADAMTS 13 replacement therapy, primarily using plasma infusion, was associated with resolution of protein- uria and a significant reduction in stroke in those receiving treatment compared to patients not on a regular regime.12 The proportion of patients on replacement therapy within the international registry was surprising. However, nearly one-third of the cohort only received treatment on demand.4 Both recent papers, therefore, raise the question of treatment in hereditary TTP. Firstly, the frequency of therapy. Despite a half-life of ADAMTS 13 of 2-4 days,13 in the international registry, trough levels were reached within 7-10 days. This has also been shown in a recent pharmaco- kinetic study in hereditary TTP.14 We need to reconsider how we treat patients, with particular attention to the dose of plasma and the frequency. The completion of the phase
I trial of recombinant ADAMTS 13, which was the first-in- human trial, using increasing doses, offers a significant advancement in the future treatment of patients with hereditary TTP, delivering a pure form of the deficient enzyme, ADAMTS 13.15
In summary, the international registry presents the impact of hereditary TTP on end-organ damage, which is evident much earlier than expected within the general pop- ulation; the most prevalent is arterial disease affecting the brain, heart and kidneys. Based on this important interna- tional collaborative study, in conjunction with other large cohorts, we must question how we should treat patients in the form of prophylactic ADAMTS 13 replacement (Figure 1). Should all patients with hereditary TTP be on prophy- laxis? Do we wait for the patient to experience frequent acute episodes before initiating therapy? Given the increased risk, particularly of arterial events after the age of 40, at what age should prophylaxis be initiated? What is the correct frequency of prophylaxis and what ADAMTS 13 activity level should we be aiming to achieve? Specific genetic variants may be the catalyst to personalized ADAMTS 13 replacement protocols. While there are many questions to be answered, without the information from important registries incorporating larger patient numbers the clinical impact of this ultra-rare disease cannot be addressed.
haematologica | 2019; 104(10)
1917