Page 211 - 2019_09-HaematologicaMondo-web
P. 211
Redox-dependent regulation of platelets
tion by protein disulphide isomerases (PDI).35 Regarding the redox-dependence of the collagen signaling in platelets, although further studies are required, protein tyrosine phos- phatases (PTP) are the most likely link between redox and conventional signaling in platelets.36 The oxidative inactiva- tion of protein phosphatases has in fact been suggested to play a key role in the activation and regulation of the patho- physiological roles of platelets.37 The Src Homology Phosphatase 2 (SHP2) has been shown to play an important role as a negative regulator of platelet activation,38 and
A
recent studies demonstrated that ROS generated during platelet activation oxidize and inhibit SHP2. This, in turns, facilitates the activation of protein kinase-mediated signal- ing pathways and drives the processes associated with cell activation (e.g. adhesion receptor activation, shape change, etc.).37,39
In this study, we also highlight the differential involve- ment of NOX1 and NOX2 in physiological agonist signal- ing (i.e. collagen and thrombin). NOX1 is essential for the signaling of collagen with NOX2 inhibition only partially
B
C
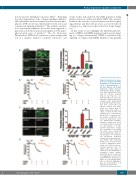
Figure 8. Experiments on trans- genic mice suggest that NOX1 and NOX2 are required for the effect of amyloid peptide β 1- 42 and oxidized low density lipoprotein (oxLDL). Platelets from wild-type, NOX1-/- or NOX2-/- mice were stained with DiOC6 and the Bioflux platform (Fluxion, San Francisco, CA, USA) was utilized to assess the thrombus formation induced by collagen under physiological flow. (A) Ibidi Vena8+ flow cham- bers were coated with 0.05 mg/mL collagen and whole blood was treated with 50 ng/mL native LDL (nLDL) or oxLDL. (C) Ibidi Vena8+ flow chambers were coated with 20 μM Aβ1-42 or scrambled con- trol peptide (ScAβ1-42). The shear rate utilized was 1,000 sec-1, which leads to thrombus formation. Images were taken at 10 minutes (min) of flow and are representative of 4 inde- pendent experiments. Images were quantified by assessing the surface area coverage by platelets with Image J. Data are representative of 4 independ- ent experiments. Statistical analysis was performed by one- way ANOVA with Bonferroni post-hoc test. *P<0.05. N=4 for (A and C). Aggregation experi- ments were performed by pre- treating platelets with 50 ng/mL native LDL (nLDL) or oxLDL (B) or 20 μM Aβ1-42 or scrambled control peptide (ScAβ1-42) (D) for 10 min. Low level aggregation was then stim- ulated with either 10 μg/mL col- lagen or 0.03 unit/mL throm- bin, as indicated. Aggregation data are representative of 3 independent experiments.
D
haematologica | 2019; 104(9)
1889