Page 17 - 2019_08-Haematologica-web
P. 17
Editorials
“Relapse” is defined by loss of MR3 [major molecular response (MMR)], and it is essential to be aware that val- ues can sometimes fluctuate between MR3 and MR4, in part because of the variability of the assay; therefore, at least two values with loss of MMR should be document- ed before therapy is resumed. This is perhaps the part of the process with the most subtleties, and consultation with CML specialists is sometimes advisable.
The pattern of relapse raises interesting issues about CML biology. Remarkably, despite the inclusion of patients with continuously undetectable transcripts for many years using very sensitive techniques, molecular relapses can be detected within the first 1-2 months of discontinuation. Virtually all relapses occur within the first 6-8 months of cessation, with very few emerging with long-term follow up which is now, in many studies, over five years. The rapidity of relapse in patients attests to the resilience of dormant CML progenitors which are capable of re-emerging almost immediately after the sup- pressive pressure of the TKI is released; it is a humbling reminder of the difficulties to be faced in eliminating stem cells in other leukemias.
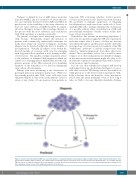
Perhaps even more fascinating is the observation of prolonged remissions lasting for many years. While it is theoretically possible that CML “stem” cells have been eliminated (Figure 1), this would seem unlikely. I am not aware of any studies of bone marrows from patients in
long-term TFR evaluating whether bcr/abl positive colonies can be grown in vitro. Interest has been shown in the possibility of immune suppression of remaining bcr/abl precursors, with some focus on the role of T-nat- ural killer (NK) cells,6,7 perhaps stimulated by observations of a possible salutary effect of proliferation of NK cells after dasatinib treatment.8,9 Results of these studies have been, at best, inconclusive.
Nonetheless, this remains an interesting hypothesis. I have seen two patients in apparent TFR who experienced molecular relapse after 1-2 years of follow up: the first after chemotherapy for another cancer and the other after prolonged use of corticosteroids for treatment of the TKI “withdrawal” syndrome. Could the relapses have been related to “immunosuppression” from these other treat- ments? This is speculative at best, but it would be inter- esting to see if other such patients are identified. Changes in the marrow microenvironment might also play a role in either the continued containment of growth or, alterna- tively, promote rapid recurrence.
It is also not clear whether late relapses will develop with longer follow up. As illustrated in Figure 1, it is pos- sible that successful TKI therapy reduces the number of CML precursors to the levels found in ‘preclinical’ CML. Little is known about the duration of the ‘incubation’ period after the initial mutagenic event or how long it takes for CML to become clinically identifiable. Perhaps
Figure 1. Chronic myeloid leukemia (CML) has a preclinical phase of unknown duration and usually is diagnosed with obvious “bulk” myeloid proliferation. After tyrosine kinase inhibitor (TKI) treatment, the cellular burden is markedly reduced to below the level of molecular detection (horizontal line). It is possible that the CML precursors are entirely eliminated (X) or tiny amounts of residual disease persist which have the potential to become clinically apparent after a long period of time, analogous to what occurs in the original presentation of the disease.
haematologica | 2019; 104(8)
1509