Page 122 - 2019_08-Haematologica-web
P. 122
S. Goossens et al.
compelling evidence, for the first time, that ZEB1 and ZEB2 can have unique functions. Overexpression in the same cellular context, using the same targeting strategy, expressed from the same endogenous promoter and in a relevant in vivo setting, resulted in clearly distinct pheno- types for ZEB1 and ZEB2. Using luciferase reporter con- structs, others have previously shown that ZEB1 and ZEB2 also have opposing effects on TGFβ1-mediated repression of the 3TP and p21 promoter elements.40 At that time, the authors hypothesized that these seemingly opposite effects were mediated by the putative differen- tial recruitment of ZEB1/2-specific co-activators/repres- sors, such as p300 and P/CAF. This differential recruitment of co-factors may specifically switch ZEB1 from a repres- sor into an activator. However, others have counteracted this hypothesis by demonstrating that ZEB1 and ZEB2 are equally potent at binding p300 and P/CAF. Furthermore, although the extensive list of ZEB-interacting proteins is growing continuously, no unique interactors have been
documented thus far. In this study, we prove that the dis- tinct phenotypes observed are not due to differences in cellular contexts or mRNA (over)expression levels. Unfortunately, no instruments are available to test whether these similar mRNA levels also translate into similar transgene protein levels. Cell-context-dependent differences in post-translational regulation of both ZEB family members may result in more or less of the ZEB1/2 transgene protein and may explain the phenotypic differ- ences observed between the two models.
It is important to mention here that although early T-cell development of R26-Zeb1tg mice is normal and these mice do not spontaneously develop leukemia, this does not exclude that other hematopoietic lineages are not affected by the Zeb1 overexpression.
In the context of T-cell malignancies, ZEB1 and ZEB2 seem to act in an opposite manner. A tumor suppressor role for ZEB1 in T-cell leukemias/lymphomas has previ- ously been suggested,41 based on its expression, the muta-
AB
C
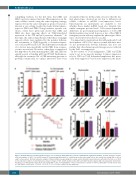
Figure 4. Zeb2-mediated T-cell differentiation delay is not essential for Zeb2-induced T-cell acute lymphoblastic leukemia formation. (A) Flow cytometric analysis of the percentages of eGFP+ cells in DN3 versus DP demonstrating differential Cre activity between the early-acting Tie2-cre line and the late-acting CD4-cre line in early T-cell precursors. (B) Flow cytometric analysis of thymi of Zeb2-overexpressing mice versus control littermates upon intercross with either the Tie2-cre or CD4- cre line. Absolute numbers of total thymocytes and percentages of CD3, CD4/8 double-positive (DP) and CD4/8 double-negative (DN) cell populations demonstrate that there is no differentiation delay in the late-acting CD4-cre line. (C) Kaplan-Meier curve for leukemia-free survival of CD4-cre, R26-Zeb2tg/tg (n=18) versus Tie2- cre, R26-Zeb2tg/tg (n=21) mice. *P<0.05, **P<0.01, ***P<0.001 (vs. control); NS, not significant.
1614
haematologica | 2019; 104(8)