Page 223 - 2019_05-HaematologicaMondo-web
P. 223
Brain to spleen stem cell migration
promise BBB function, enabling cells such as macrophages to pass the barrier.7 Thus, while our data demonstrate that hBMSC likely utilized lymphatic vessels to migrate to the spleen, it is also possible that hBMSC in the brain were able to cross the now permeable BBB and enter the blood- stream, enabling them to migrate to the spleen via sys- temic circulatory pathways. As the groups with severe stroke had more severe inflammation, this may have resulted in more damage to the BBB, and could also explain why more transplanted cells migrated from the brain to the periphery (i.e., spleen) in these groups. Future studies could further probe this concept by measuring stem cell levels in the blood, as well as graft deposition in other peripheral organs.
The in vitro results revealed an increase in hBMSC migra- tion toward lymphatic endothelial cells, microglia, or a
combination of both when treated with TNF-a. The increasing number of migratory hBMSC was dependent on the dose of TNF-a in every experimental group. Interestingly, the greatest migratory activity was seen in the cultures of TNF-a-treated microglia cells alone versus either lymphatic endothelial cells or the co-culture of both cell types with TNF-a. Since bEnd.3 cells are a brain endothelioma cell line, it is possible that the hBMSC also migrated to brain parenchymal endothelial cells, in addi- tion to LYVE-1 lymphatic endothelial cells. However, adding TNF-a increases the ability of bEnd.3 cells to form LYVE-1-expressing lymphatic tubes.50 Thus, it is likely that in our cell migration assays involving TNF-a, the majority of hBMSC migrated to the LYVE-1 lymphatic endothelial cells, which were likely present in a higher proportion.
Prior studies have demonstrated that the injection of
AC
BD
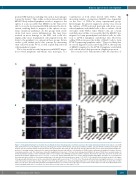
Figure 6. Transplanted human bone marrow mesenchymal stromal cells phagocytose ischemic neurons in the brain and transport them to the spleen. (A and B) Triple immunofluorescent staining for human specific phagocytic marker CD68 (CD68), anti-apoptosis inhibitor 5 (API5), and anti-160kD neurofilament medium anti- body-rat specific neuronal marker (neuronal marker) was performed in the brain (A) and spleen (B). Groups with mild and severe stroke demonstrated higher fre- quencies of staining overlap compared to the sham-treated group. Arrow heads indicate co-localization of CD68-positive, anti-apoptosis inhibitor 5-positive, and neu- ronal marker-positive cells. The small boxes show 40x magnification. Scale bars = 100 mm. Red: anti-apoptosis inhibitor 5; green: CD68; blue: neuronal marker. (C and D) Quantitative analyses of the estimated number of co-localized cells exhibiting overlap for all three stains in the brain (C) and in the spleen (D) of stroke and sham-treated animals. Significance bars: **P<0.01; ***P<0.001. Groups with mild and severe stroke displayed significantly higher quantities of co-localization in the brain and spleen relative to the sham-treated group on all days that measurements were made (**P<0.01). Co-localization levels peaked on day 3 in all organs in the groups with mild and severe stroke, especially in the groups with severe stroke (**P<0.01). (C) a: The group with mild stroke had significantly more co-localized cells in the brain on day 3 than on other days (P<0.05); b: the group with severe stroke had significantly more co-localized cells in the brain on day 3 than on other days (P<0.01). (D) a: The group with mild stroke had significantly more co-localized cells in the spleen on day 3 than on other days (P<0.05); b: the group with severe stroke had significantly more co-localized cells in the spleen on day 3 than on other days (P<0.05).
haematologica | 2019; 104(5)
1069