Page 174 - 2019_04-Haematologica-web
P. 174
S.E. Sylvan et al.
line CLL treatment in the period 2007-2013. As office- based private medicine is practically non-existent for CLL in Sweden, all patient files were identified, ensuring high- quality data and minimal selection bias, which is a key strength of our report. The time period 2007-2013 was selected in order to obtain sufficient follow up.
Subjects in this study were older than in previous clini-
cal studies,6,8,32,33 more often had advanced disease but were in good performance status. The median age in our cohort is consistent with median age at diagnosis,3 and the advanced stage combined with good performance sta- tus reflects treatment indication and first-line treatment status. The majority of patients were treated in the later time period which possibly suggests that most patients
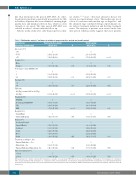
Table 3. Multivariate analysis* on factors in relation to progression-free survival and overall survival.
Clinical factor Progression-free survival Overall survival
(percentage available data) HR (CI 95%) P HR (CI 95%)
Age, years (100)
<65 1 1
P
<0.001
0.016
0.001
0.263
<0.001
0.010
0.936
0.003
0.989
0.523
65-74 1.41 (1.02-1.93)
>75 1.86 (1.24-2.80)
Gender (100)
Males 1
Females 0.70 (0.53-0.94) Performance status (ECOG) (98)
2.30 (1.39-3.79) 0.010 3.76 (2.12-6.67)
1
0.017 0.60 (0.40-0.91)
011
1 1.60 (1.18-2.18) 2.33 (1.48-3.66) 2-3 1.87 (1.12-3.13) 0.006 2.25 (1.10-4.59)
Rai stage (98)
0-2 1 1
3-4 FISH (64)
del(13q), normal, tri12 or del(11q)
del(17p)
Treatment (99)
F/FC/FCR Alemtuzumab/CHOP/CVP CLB
B/BR
Hospital (100) County/Rural University/Regional
Region (100)
Stockholm/Gotland Uppsala/Örebro Southeast
South
West
North
Treatment according to: (99)
National Guidelines – Yes
Clinical trial – Yes
National Guidelines/Clinical trial - No
Time to treatment (100)
0.92 (0.71-1.19)
1
2.05 (1.43-2.91)
1
1.68 (1.09-2.57) 2.82 (1.86-4.26) 0.79 (0.47-1.33)
1
0.82 (0.61-1.09)
1
0.78 (0.51-1.19) 1.03 (0.65-1.63) 1.22 (0.78-1.92) 0.97 (0.59-1.60) 0.93 (0.54-1.62)
1
1.80 (1.07-3.04) 1.14 (0.72-1.81)
0.53
<0.001
<0.001
0.166
0.481
0.082
1.22 (0.86-1.75)
1
2.17 (1.38-3.39)
1
1.32 (0.74-2.37) 2.40 (1.43-4.04) 1.14 (0.56-2.33)
1
1.02 (0.69-1.50)
1
0.80 (0.43-1.49) 1.62 (0.85-3.08) 2.42 (1.32-4.39) 2.21 (1.13-4.33) 1.46 (0.70-3.07)
1
1.02 (0.45-2.28) 1.05 (0.58-1.89)
<1year 1 1
≥1 year 0.94 (0.72-1.23) 0.652 0.89 (0.63-1.27)
HR: hazard ratio; CI: confidence interval; ECOG: Eastern Co-operative Oncology Group; FISH: fluorescence in situ hybridization; F: fludarabine; FC: fludarabine in combination with cyclophosphamide; FCR: fludarabine in combination with cyclophosphamide and rituximab; alemtuzumab/CHOP/CVP: alemtuzumab /cyclophosphamide+hydroxy- daunorubicin+vincristine+prednisone/cyclophosphamide+vincristine+prednisone; CLB: chlorambucil; B/BR: bendamustine/bendamustin and rituximab. *Model included all listed factors.
802
haematologica | 2019; 104(4)