Page 20 - 2019_01-Haematologica-web
P. 20
10
Editorials
with CLL.3 The patients enrolled in this trial were consid- ered ineligible to receive the combination of fludarabine, cyclophosphamide, and rituximab (FCR) because of their older age or the presence of comorbidities. For the first six cycles (induction-I), lenalidomide was given in combination with chlorambucil and rituximab at a starting dose of 2.5 mg, with escalation to 10 mg. The authors report that they were able to administer a median lenalidomide dose of 86.7% of the full dose, with the full dose given to more than 50% of patients. For the next six cycles (induction-II), lenalidomide was given as monotherapy at a dose of 10 mg daily. The median administered dose was 99.7% of the full dose, and the full dose was given to 69% of patients during
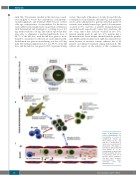
A
cycle 6. The results of this phase 1-2 study showed that the combination of lenolidamide, chlorambucil, and rituximab can be safely administered to patients with CLL: grade 3-4 toxicities were mainly hematologic (grade 3-4 neutropenia occurred in 73% and 64% of patients during induction-I and induction-II, respectively), tumor lysis syndrome did not occur, tumor flare reaction occurred in five (9%) patients (mainly grade 2), and two (4%) patients had a thromboembolic event despite thromboembolic prophy- laxis. Of 53 patients in induction-I, eight discontinued treat- ment because of excessive toxicity, whereas five of 42 patients discontinued treatment during induction-II. The authors also report on the activity of this combination:
B
C
Figure 1. Mechanisms of action of lenalidomide. The mechanisms of action of lenalidomide include: (A) direct effects on CLL cells and (B, C) modification of tumor-microenvironment interactions. This figure is reproduced with permission from Mattei R et al., Lenalidomide in chronic lym- phocytic leukemia: the pres- ent and future in the era of tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 206;97:291-302. NLCs: nurse-like cells; EC: endothelial cells.
haematologica | 2019; 104(1)