Page 193 - 2018_11-Haematologica-web
P. 193
Glycosylation modulates FVIII immunogenicity
observed no difference in the binding of BHK- or CHO- rFVIII to naïve splenocytes or DCs. Moreover, the removal of a2-3, and a2-6 sialic acids did not significantly influ- ence cellular binding or modulation of surface co-stimula- tory molecule responses in vitro, and did not influence the immunogenicity of BHK-rFVIII in mice. In fact, the FVIII immune response was attenuated when exposed to desia- lylated BHK-rFVIII compared to exposure to unmodified rFVIII, potentially due to desialylated BHK-rFVIII behav- ing as a unique antigen.47 Although the antigenicity of desialylated rFVIII was not altered as determined by
A
ELISA, the decrease in FVIII:C activity suggests an alter- ation of global tertiary structure that may influence FVIII immunogenicity.
Interactions between FVIII and its plasma binding part- ners may regulate its association with endocytic cells in the liver and spleen. We observed increased binding to BHK-rFVIII by non-neutralizing IgM in the plasma of naïve HA and HA-R593C mice as well as by non-neutral- izing IgM and IgG from healthy human subjects. The presence of mouse and human VWF in these experiments suggests that these immune complexes can form in the cir-
B
CD
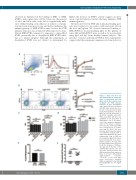
Figure 6. Sialic acid does not alter FVIII binding to, and matu- ration of splenocytes and den- dritic cells in vitro, and does not modulate the FVIII immune response in vivo. Desialylated and control rFVIII were incubated with FVIII naïve splenocytes for 1 hr at 4°C and assessed for bind- ing by flow cytometry. Cells were gated as total (A) splenocytes or (B) CD11c+ dendritic cells (DCs). rFVIII association with DCs was quantified as the proportion of FVIII+CD11c+ out of all CD11c+ cells. Flow cytometry characteri- zation of the surface co-stimula- tory molecules, CD80 and CD86, were performed on (C) spleno- cytes and (D) CD11c+ DCs incu- bated for 24 hr under the indicat- ed conditions (1 mg/mL LPS or rFVIII; n=4). HA-R593C mice were administered 1 mg of rFVIII and its desialylated glycoform subcu- taneously biweekly for 2 weeks. Plasma samples 28 days after the initial infusion were assessed for (E) the incidence of FVIII-spe- cific IgG and (F) the titre of the anti-FVIII IgG response. Line and error bars represent mean and SEM respectively. Data represen- tative of at least 3 independent deglycosylation reactions. **P<0.01; ***P<0.001. MFI: mean fluorescence intensity; rFVIII: recombinant factor VIII; Ig: immunoglobulin; n.s. not signifi- cant.
EF
haematologica | 2018; 103(11)
1933