Page 14 - 2018_11-Haematologica-web
P. 14
Editorials
hepcidin exposure. Based on both the clinical phenotype and functional tests the variant was assigned as a hepcidin sensitive loss-of-function variant. In the open outward con- formation of the 3D structure, using Bdellovibrio bacteriovorus Bd2019 as the template, the demonstrated non-covalent interaction between Arg178 and Asp473 (located on the N- and C-lobe, respectively) was assumed to be involved in the stabilization of the open outward conformation needed to preserve iron egress. Indeed, the Asp473Ala ferroportin
variant was also properly expressed on the membrane with a nearly total loss of iron export capacity. In most loss-of- function variants the abolished iron transport is attributed to defective expression of ferroportin on the membrane. Ka et al., however, provide evidence for interference in the sta- bilization of the conformation state, i.e., the open outward state, in the Arg178Gln variant as an alternative mechanism for diminished iron transport, despite the proper membrane expression of this variant. Further exploration of the amino-
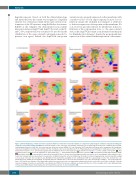
Figure 1. Model displaying cellular iron flows in patients with loss-of-function ferroportin variants as compared to physiological conditions and patients with gain- of-function ferroportin variants and IRIDA. Ferroportin activity in the enterocyte, macrophage and hepatocyte in physiological steady state conditions (A), hepcidin- resistant gain-of-function (GOF) (B), iron refractory iron deficiency anemia (IRIDA) (C), loss-of-function (LOF) with mislocalisation on the membrane (D), membrane expressed destabilized and hepcidin sensitive LOF (E), membrane expressed destabilized LOF (F). Physiologically, iron availability for erythropoiesis (in the form of transferrin bound iron ( ) is systemically regulated by the inhibitory effects of the hepatocyte-derived hormone hepcidin ( ) on the activity of ferroportin ( ). This regulatory system contributes to the regulation of intracellular iron ( ) and ferritin bound iron ( ) levels, serum iron ( ) concentration, transferrin saturation (TSAT) ( ) and serum ferritin ( ) concentration. In hepcidin-resistant GOF, there is an increased non-impressionable iron export out of macrophages with continuous ente- rocyte iron transport with iron deposition in hepatocytes. In IRIDA, ferroportin action is inhibited by (for body iron status) inappropriately elevated hepcidin leading to decreased enterocyte iron resorption and diminished ferric-transferrin availability for the erythroblast with the sequestering of some iron, derived from ery- throphagocytosis, within the macrophage. In patients with LOF variants there is decreased iron transport out of the macrophage leading to iron sequestration in these cells, but the mechanism of the apparently relatively increased enterocyte iron export with subsequent body iron overload is not yet fully elucidated. Clinically observed changes in serum iron, and ferritin concentration and TSAT in the various conditions are depicted by the size of the rectangles in the lower right-hand side of the panels. DMT: divalent metal transporter; tfr: transferrin receptor-1; fe: iron. Transferrin ( ).
1754
haematologica | 2018; 103(11)