Page 48 - 2018_09-Mondo
P. 48
M.S. Talkhoncheh et al.
AB
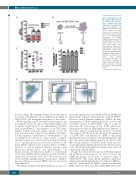
Figure 3. Targeting of the NF- κB regulator IKKβ improves the in vitro and in vivo func- tion of cultured cord blood (CB)-derived hematopoietic stem and progenitor cells (HSPCs). (A) Colonies per 300 CD34+ input cells were scored after 14 days (n=3). (B) Schematic representation of in vivo transplantation
CD
experiment. (C) engraftment was assessed by quantifying the percentage of human CD45+ cells in the bone marrow (BM) of NSG recipients four months post transplantation (data from 2 independent experiments). (D) Lineage distribution was quantified by FACS analysis of human B cells (CD19), T cells (CD3), and myeloid (CD33/CD15) in CD45+ cells of the BM of recipients. (E) Representative FACS plots for gating strategy of lineage reconstitution. PF: PF184; TP: TPCA1.
Human
E
tial after culture. Our findings further showed that this is associated with inhibition of pro-inflammatory signals in CD34+CD90+ cells during the early phases of the culture.
molecular signature associated with sh758, an shRNA that dramatically expands phenotypically defined HSPCs. However, even if chemical inhibition of IKKβ, the inte- grating kinase of NF-κB signaling, did lead to a robust and consistent increase in CD34+CD90+ cells, it could not reproduce the full extent of the sh758 phenotype. Specifically, the effect of IKKβ inhibition was limited to a short time window during the first 24 hours of culture. This suggests that the profound and persistent expansion of CD34+CD90+ cells induced by sh758 is only partly mediated by reduced activity of the NF-κB pathway, and that other genes and pathways must be involved as well. One strong candidate is MAPK14 (p38), which was also down-regulated in sh758-transduced cells. We have previ- ously shown that p38 inhibition enhances the stem cell output from cultured HSPCs.
NF-κB signaling can be activated in both immune and non-immune tissues by various extracellular signals, such as reactive oxygen species,21 pro-inflammatory cytokines such as interleukin-1,22 and members of the TNF super- family.23 Cellular responses largely depend on the cell type, include positive or negative modulation of prolifera- tion and apoptosis,16 differentiation,24 development,13,25 and are mediated by secretion of a large variety of signals21 and direct expression control of cell cycle/apoptosis medi- ators.16 In hematopoiesis, knockout mouse models of dif- ferent NF-κB subunits have revealed defects in HSPCs function, as well as impaired immune response, lym- phopoiesis, granulocytosis, and splenomegaly.26,27 In human settings, overexpression of p65 or a constitutively active form of IKKβ did not influence growth and differ- entiation of CB-derived CD34+ cells.28 However, the actu- al role of NF-κB signaling and the consequences of inhibit- ing the pathway in normal HSPCs had not previously been addressed.
Despite the well described function of NF-κB in regulat- ing proliferation and apoptosis,29 we did not observe any significant changes in cell cycle, or apoptotic status of CD34+CD90+ cells upon NF-κB pathway inhibition. Additionally, NF-κB signaling has been linked to ROS pro- duction,21 which must be tightly regulated to maintain HSPC function.30 However, intracellular ROS levels were
We initially identified the NF-κB pathway as part of a
1448
haematologica | 2018; 103(9)